Research Highlights(Organic and Biological Chemistry)
- Organic Structural Biochemistry Laboratory
- Organic Chemistry Laboratory
- Biochemistry Laboratory
- Synthetic Organic Chemistry Laboratory
Organic Structural Biochemistry Laboratory: Visualise the 3D structures and dynamics of proteins and DNA in physiological environments such as in live cells
Biomacromolecules such as proteins exhibit various functions by maintaining their unique three-dimensional (3D) structures and yield complicated biological phenomena by which they properly gather and communicate with each other. Therefore, it is essential to elucidate the conformations of biomacromolecules and their interactions in order to understand the fundamental mechanisms of living systems and diseases at the molecular level and lead the development of new drugs. Nuclear Magnetic Resonance (NMR) spectroscopy is attracting attention as the only method that can analyse molecular structures at atomic resolution even under near-physiological conditions. In our laboratory, we are developing new NMR measurements and analysis techniques for in-situ observations of the biomacromolecules in living cells (in-cell NMR) and studying its applications.
Organic Chemistry Laboratory: Design of Molecular Catalysts Directed Toward Green Sustainable Chemistry, Circular Economy
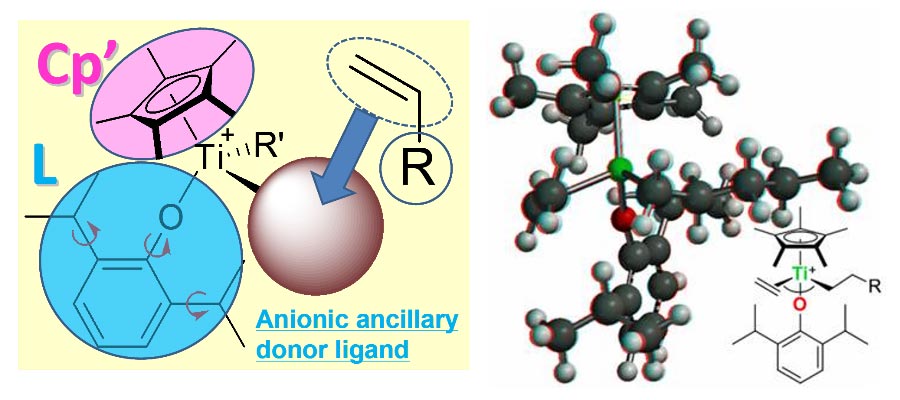
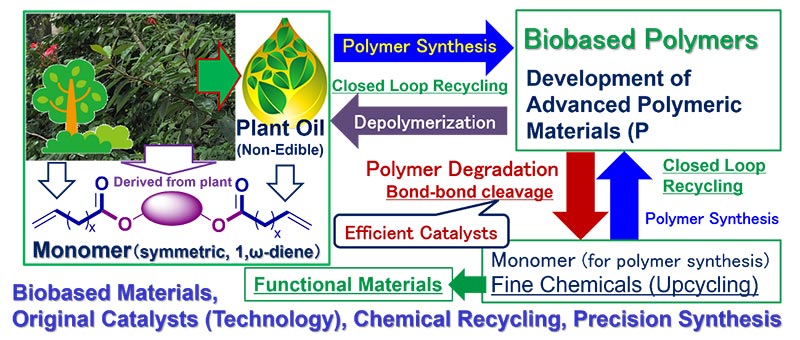
Subject fields in the Organic Laboratory, Department of Chemistry, Tokyo Metropolitan University (TMUORG) is Organometallic Chemistry, Molecular Catalysis, Polymers and Materials Chemistry. Ongoing subjects in our laboratory are (i) design of efficient molecular catalysis for precise synthesis of organic molecules as well as polymers for green and sustainable chemistry, (ii) design and synthesis of functional materials by precise synthesis. We also conduct subjects concerning (iii) synthesis and reaction chemistry of highly reactive organometallic complexes/intermediates for efficient carbon-carbon bond formation (insertion, metathesis, coupling etc.), (iv) development of recyclable bio-based polymers and their chemical recycling, upcycling by efficient bond formation/cleavage, and(iv) precise synthesis of π conjugated oligomers/polymers for advanced materials.
Subject fields: Organometallic Chemistry, Molecular Catalysis, Homogeneous Catalysis, Organic Synthesis by Organometallics, Materials Chemistry (Functional Polymers, Conjugated Oligomers/Polymers)
Selected Publications (Perspectives, Reviews)
(1) “Synthesis of vanadium-alkylidene complexes and their use as catalysts for ring opening metathesis polymerization”
K. Nomura, X. Hou, Dalton Trans., 46, 12-24 (2017). Perspective(2) “Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET)”
Y. Chen, M. M. Abdellatif, K. Nomura, Tetrahedron (Report), 74, 619-643 (2018).
(3) “Solution XANES and EXAFS analysis of active species of titanium, vanadium complex catalysts in ethylene polymerisation/dimerisation and syndiospecific styrene polymerisation”
J. Yi, N, Nakatani, K. Nomura, Dalton Trans., 49, 8008-8028 (2020).
(4) “Organometallic complexes of group 5 metals with metal-carbon sigma and multiple bonds”
K. Nomura, Comprehensive Organometallic Chemistry IV, Elsevier, vol. 5, p. 587-650 (2021).
(5) “Synthesis of bio-based aliphatic polyesters from plant oils by efficient molecular catalysis: A selected survey from recent reports”
K. Nomura, N. W. Binti Awang, ACS Sustainable Chem. & Eng., 9, 5485-5505 (2021).
Biochemistry Laboratory
Molecular Mechanisms of Chromosome Maintenance
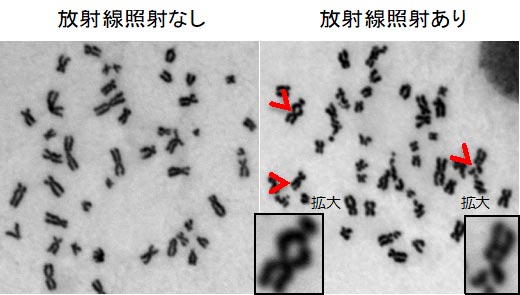
Human genetic information is written on a molecule called DNA. The entire human genome is approximately 3 billion letters of ACGT, which mean a total of 2 meters long DNA molecule in each cell. DNA is stored in a highly ordered structure as chromosomes in the tiny nucleus, which is about 10 μm in diameter (Figure 1 is an image of chromosomes). How is our chromosomal DNA copied (replicated), repaired and inherited by our children? We are studying the regulation of such the chromosome maintenance mechanisms of repair, replication, and inheritance. It is known that inactivation of these chromosomal maintenance mechanisms leads to “cancer”. We are trying to propose new cancer therapies based on the knowledge obtained through our research.
Ribonucleoproteome Research
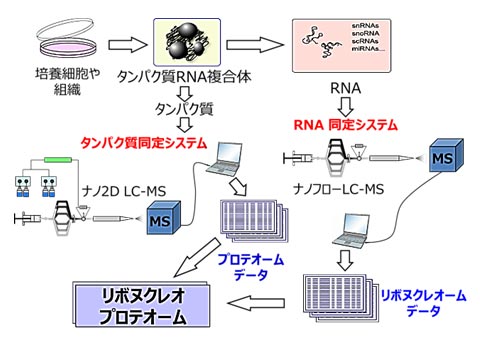
Advances in genetic analysis technology are revealing the full extent of the information encoded in the “genome,” which can be considered the blueprint for humans and other living organisms. From this genomic information, we know, for example, that 21,000 types of proteins and many types of RNAs are at work during a human’s lifetime. So which proteins and RNAs are at work at a particular moment in a human lifetime, and how do they interact with each other? By accumulating answers to this question, challenging research has begun around the world to uncover the mechanisms of life. Just as the set of genes is called the genome, the set of proteins and RNAs actually at work in the cells of the brain and liver is called the ribonucleoproteome. We are developing state-of-the-art LC-MS technologies to analyze the entire ribonucleoproteome and its functional network by combining mass spectrometry with genomic information. Through research using these technologies, our goal is to understand the mechanisms of life and its abnormalities in the “language of molecules”, mainly proteins and RNAs, and to provide new medical technologies that target these molecules.
Laboratory of Synthetic Organic Chemistry: Design of Electronic States and Reactions Based on the Characteristics of Main-Group Elements
Our laboratory focuses on the atomic states and bonding situations of molecular materials to realize molecules with unique electronic configurations and geometric structures that have never been achieved before. In particular, we are trying to develop new aspects of elements by taking advantage of the characteristic features of main-group elements such as boron, silicon, and oxygen etc. and with precise design of the bonds between carbon and a main-group element or bonds between a transition metal and a main-group element. We are also working on the development of characteristic “functions” derived from the unique electronic structure of synthesized molecules and the development of new “reactions” to cleave and form bonds.
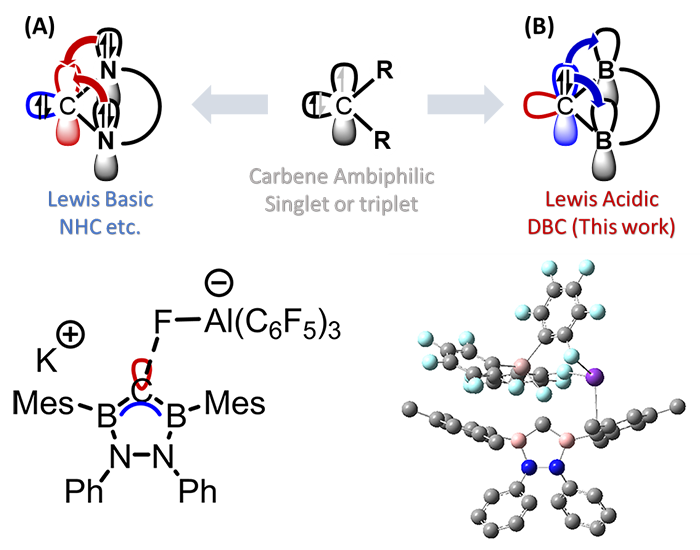
Realization of unique electronic configuration by designing π-bonding orbitals
Carbon is a group 14 element and normally forms four bonds. The singlet carbene has electrons in the in-plane sp2 hybrid orbital, and the p-type orbital is kept empty. The substitution of π donating elements such as nitrogen stabilizes this electronic state and is used in a variety of fields (Figure 1, upper A). We have synthesized a molecule consisting of a carbene carbon bearing two neighboring boron atoms (DiBorylCarbene) and achieved to reverse the empty and occupied orbitals of the carbene carbon (Figure 1, upper B, and bottom). Diborylcarbene exhibits an extremely strong Lewis acidity and is expected to have a distinctive coordination chemistry even though it is a neutral carbon species.
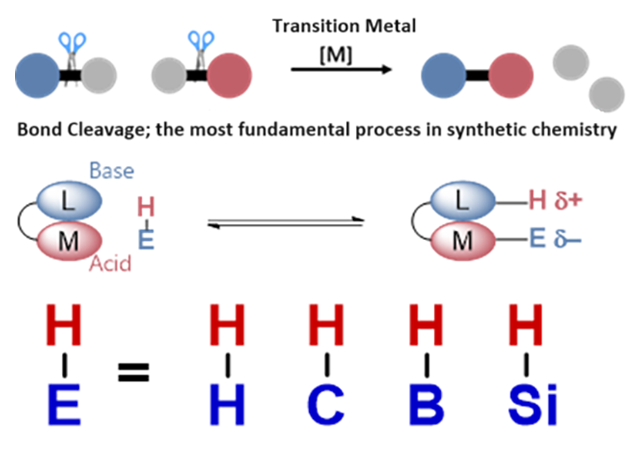
Metal-ligand cooperative bond breaking/formation
The elementary reaction processes of bond cleavage and formation are the most fundamental processes in synthetic organic chemistry for making molecules. In recent years, transformation reactions involving bond cleavage using transition metal complexes have been actively studied and have become indispensable. We are developing new molecular transformation technologies by focusing on “metal-ligand cooperative function,” in which not only the metal center but also the organic ligand coordinated to the metal simultaneously and cooperatively cleave and form bonds. By precise design of the ligand and controlling the transfer of electrons between the metal and the ligand, we are trying to control reactions and selectivity that have never possible before.