Research Highlights(Physical Chemistry)
- Solid State Physical Chemistry Laboratory
- Physical Chemistry of Molecular Structure and Reaction Laboratory
- Theoretical and Computational Chemistry Laboratory
Solid State Physical Chemistry Laboratory: Development of Transparent Electrode for Next Generation Optoelectronics Devices
Our group is working on the development of solid materials. In particular, we focus on the unique functions and physical properties of materials of which crystal structures or chemical compositions cannot be obtained by the conventional synthesis technic of solid compounds (mixing and heating of raw powder materials). For this purpose, thin film synthesis methods such as pulsed laser deposition, in which a pellet of raw materials in a vacuum chamber is vaporized by laser irradiation and deposited on a substrate, and mist chemical vapor deposition, in which tiny droplets of a solution including raw materials react on a substrate (Fig. 1). We would like to introduce a highlight or our recent research regarding the development of a material known as a “transparent electrode”.
Optoelectronic devices that convert light into electricity or generate and control light with electricity, such as photovoltaic cells, light-emitting diodes (LEDs) and liquid crystal displays, are indispensable in our daily lives. In most of these devices, materials known as “transparent electrodes”, which exhibit both high electrical conductivity and high optical transparency, are used as windows to introduce or extract the light.
Currently, thin films (several 10 nm to several 100 nm thick) of tin-doped indium oxide and antimony- or fluorine-doped tin oxide are widely used as commercial transparent electrodes. These materials are transparent to visible light but absorb or reflect ‘invisible’ light such as infrared and ultraviolet. This makes them unsuitable for application in next-generation optoelectronic devices such as high-efficiency solar cells utilizing even the infrared in sunlight to generate electricity and ultraviolet LEDs, which are expected as the alternative light source of mercury lamp for disinfect and virus detoxification.
We have recently found that a crystalline thin film of tin oxide doped with a small amount of tungsten can be used as an electrode material that is transparent against near-infrared as well as visible light. Using synchrotron radiation X-rays to study the crystal structure on an atomic scale and theoretical calculations to predict the state of the tungsten, we have revealed that the interaction of electron orbitals of the doped tungsten ions and the surrounding oxygen ions in the crystals causes an unconventional valence state of the tungsten, inducing the optical transparency against near-infrared.
We also discovered that atomic-scale mixture of tin oxide crystal with germanium oxide one (“mixed crystal”) can be used as electrodes transparent to ultraviolet radiation. Intriguingly, when tin oxide and germanium oxide powders are heated to react with each other, they separate into their individual components, but a pulsed laser deposition method, in which atomically resolved raw materials are reacted at low temperatures, can synthesize uniformly mixed crystals. A transparent electrode with the world’s highest level of performance has been achieved by adding a small amount of tantalum to this mixed crystal (Figure 2).
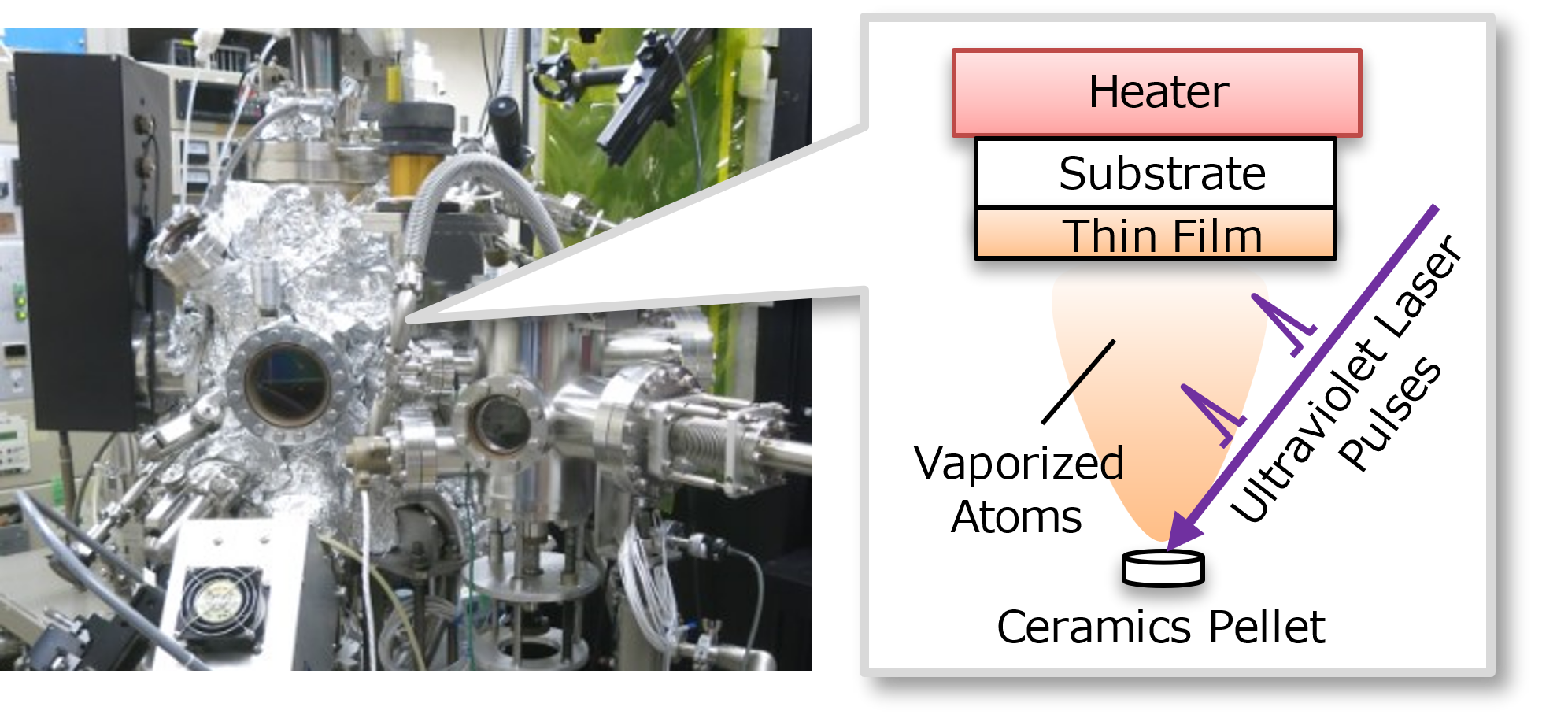
Caption: Photograph of the pulsed laser deposition system and schematic illustration of the thin film growth process.

Caption: Photograph of the deep ultraviolet-transparent electrode (SGO, right) and a conventional material (SnO2, left) on white paper illuminated by ultraviolet light. Bright luminescence from the paper under the SGO is observed because of its high transparency against ultraviolet. On the other hand, no luminescence is observed under the SnO2 because the ultraviolet is totally absorbed by the SnO2.
Physical Chemistry of Molecular Structure and Reaction Laboratory:
Slow-motion filming of chemical reactions
A single molecule is only about one-millionth of a millimeter in size, however, as we learned in junior high and high school chemistry, we know its “shape” very well. This is because the structures of various molecules have been measured with high precision using an experimental technique called gas electron diffraction. In this technique, a molecule is irradiated with an electron beam, which has quantum mechanical wave properties, and the interference pattern of the scattered electron wave is photographed for measuring the molecular structure. The exposure time of the conventional gas electron diffraction method is from several tens of seconds to several minutes, and consequently, objects (molecules) in motion have been photographed blurredly.
On the other hand, recently, a new cutting-edge experimental technique called gas pulsed-electron diffraction has been developed, which can precisely measure an “instantaneous” structure of molecules undergoing chemical reactions. In this technique, instantaneous molecular structures can be measured by irradiating electron beam pulses with short durations like camera flashes. Using this method, several attempts were conducted to capture slow-motion movies of molecules undergoing chemical reactions. However, the temporal width of the electron beam pulse was limited to around 100 femtoseconds (1/10 trillionth of a second), which was not enough for obtaining a slow-motion movie of chemical reactions without blurring because the typical time scale of molecular structural changes is in the order of 10 femtoseconds (1/100 trillionth of a second).
In order to drastically improve the temporal resolution, we proposed a laser-assisted electron diffraction (LAED) method.1 In the LAED method, we utilized a phenomenon called “laser-assisted electron scattering” (LAES) as an ultrafast shutter, in which the kinetic energy of electrons changes by an integral multiple of the photon energy of the laser field through the scattering by atoms or molecules in a laser field (Fig. 1). Since the LAES process occurs only in the presence of a laser field, the instantaneous molecular structures can be revealed through the analyses of the energy-shifted scattered electrons even if an electron beam with a long duration is used (Fig. 2). Since the temporal width of the laser pulse can be shorter than 10 femtoseconds, the LAED method can achieve a time resolution of less than 10 femtoseconds, which is enough to capture the slow-motion movie of chemical reactions without blurring. By realizing this unique experimental method,2 we are trying to visualize how molecules change their shape in chemical reactions.
[2] Y. Morimoto, R. Kanya, K. Yamanouchi, J. Chem. Phys. 140, 064201 (2014).
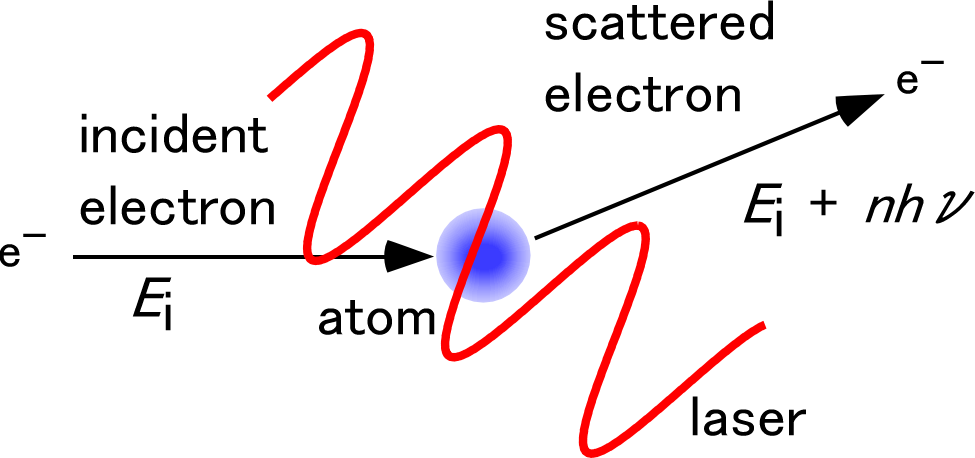
Caption: Schematic of the LAES process. Through the LAES process, the incident electron energy (Ei) is changed by an integer multiple of the photon energy (hν), and becomes Ei + nhν.
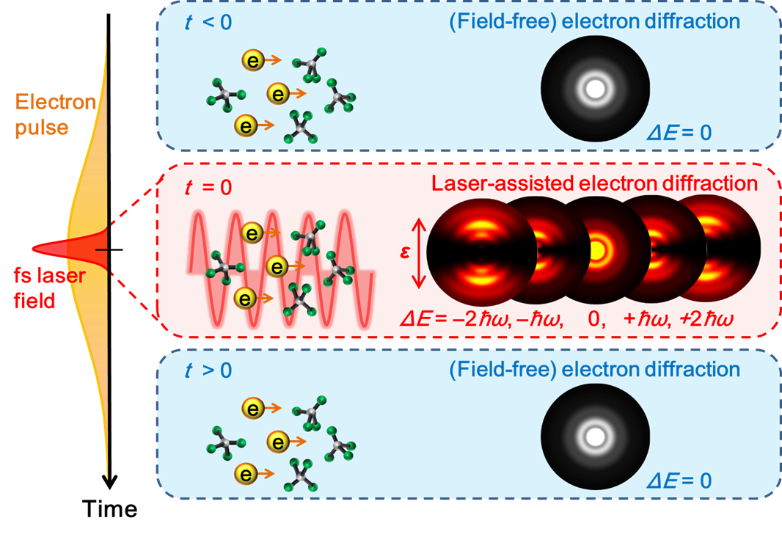
Caption: Principle of the LAED method. Since only the scattered electrons that collide with the molecule at the moment when the electron beam pulse and the laser pulse coexist change their energy (ΔE ≠ 0), analyzing the energy-shifted scattered electrons yields the instantaneous molecular structure at the moment of the laser pulse irradiation.
Theoretical and Computational Chemistry Laboratory:
Theoretical and computational chemistry in transition metal complex systems
Transition metal complexes and metal nano-clusters are important research subjects in chemistry, including catalytic chemistry, materials chemistry, and so on so forth. Particularly, compounds containing the 3d transition metal elements such as Fe, Ni, and Cu, etc., have complicated electronic structures due to strong electron correlation effect from 3d electrons, which are very difficult to characterize both experimentally and theoretically. In our research group, we are working on theoretical chemistry and computational chemistry of such 3d transition metal elements to investigate physical and chemical properties of various metal complexes, crystals, and surfaces.
Recently, in collaboration with Nomura group (TMU) and Yamazoe group (TMU), we have conducted computational simulations for the K-edge XANES spectra of various 4-5 group complexes which are model complexes of the alkene polymerization catalysts. Since the spectral shape of the XANES spectra highly depends on the valence electronic structure, we have performed the simple TDDFT calculations to quantitatively reproduce the experimental XANES spectra (Fig. 1), and succeeded to characterize the active species in the catalytic reaction.
In collaboration with Kato group (Kwansei Gakuin University), we have theoretically investigated the vapochromic crystal of Ni-quinonoid complex. Especially, we have predicted unknown crystal structure of the Ni complex to reveal the electronic mechanism of vapochromism (Fig. 2). Of considerable interest in this molecule is, that the 1D band structure appears due to intermolecular interaction, which disappears upon absorption of a guest molecule to change the color of crystal.
On the other hand, we are also working on development of a new theory to accurately compute the complicated electronic structure of 3d transition metal elements. In the electronic system which exhibits strong electron correlation effect, it is known that a multi-reference character appears in the electronic wavefunction. This leads serious deterioration in computational accuracy of methods based on the 1-electron approximation (such as the Hartree-Fock theory and Kohn-Sham DFT method). Thus, the multi-reference method, such as CASSCF/CASPT2 method, is required even for qualitative discussions. However, due to the strong arbitrariness in the choice of CAS space, a robust and accurate method for the strongly correlated system has not yet been established. In our research group, we aim to construct a new multi-reference method which does not depends on the choice of CAS space, by dividing the full configuration space with the seniority number.
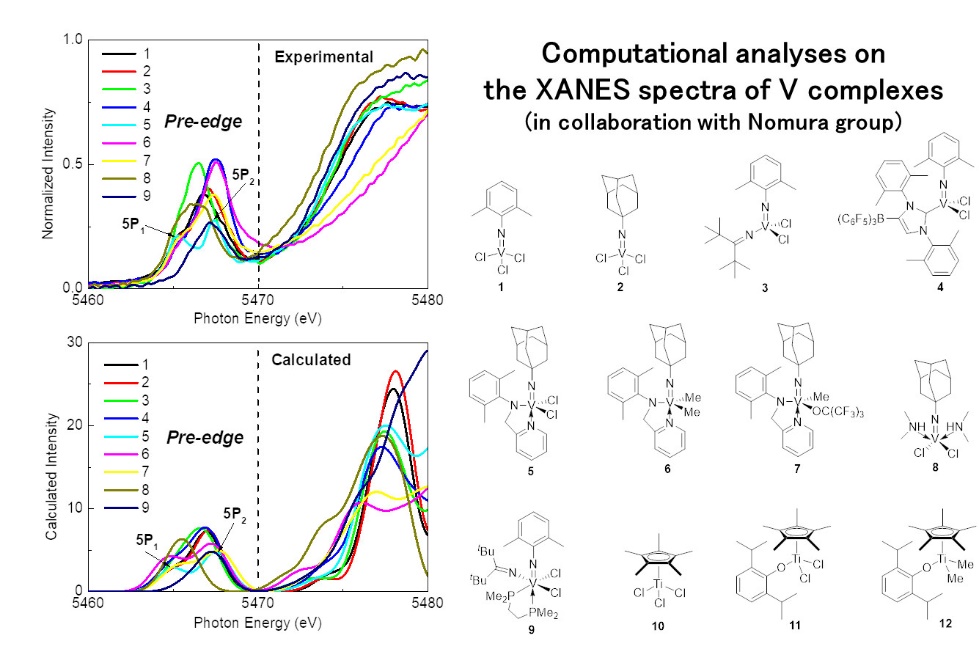
Caption: Experimental and computational XANES spectra of various V complexes (Phys. Chem. Chem. Phys. 2020, 22, 674-682).
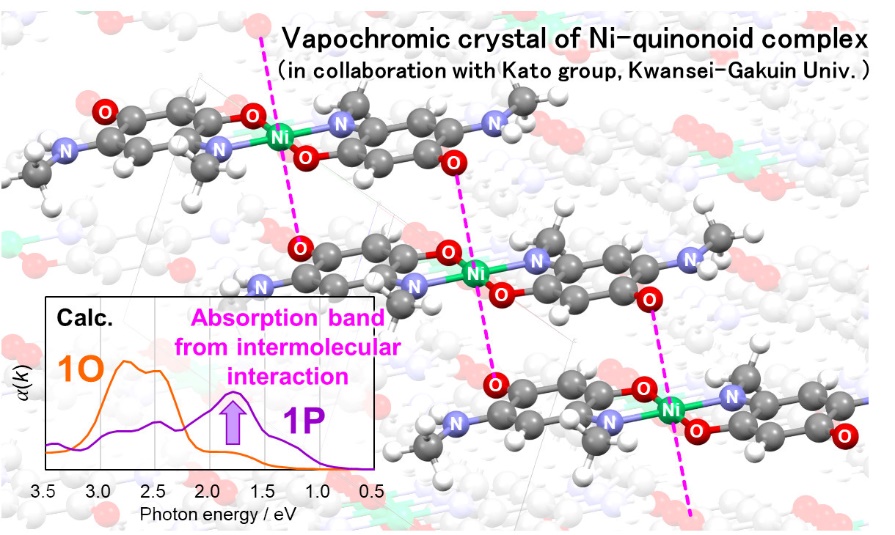
Caption: Predicted crystal structure and simulated UV spectra of the Ni-quinonoid complex


