Research Highlights (Inorganic and Analytical Chemistry)
- Coordination Chemistry Laboratory
- Environmental Geochemistry Laboratory
- Inorganic Chemistry Laboratory
- Isotope Chemistry Laboratory
Coordination Chemistry Laboratory : Development of photo-functional molecular materials based on metal complexes
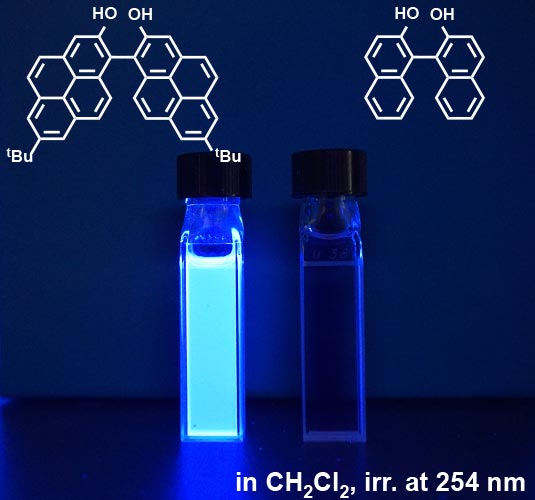
Both normal gloves and work gloves protect our hands from the cold and dirt. These two gloves are similar, but what is the decisive difference between them? Normal gloves have a right-hand and a left-hand, but work gloves have no such distinction. There are also molecules for which the right and left can be distinguished and expressed as “chiral molecules”. Chiral molecules have various characteristics, and their response to light is the most important. “Chiral molecules” are sometimes referred to as “optically active substances”.
However, among molecules, some substances emit light called fluorescence. Fluorescence refers to the fluorescence in a fluorescent pen. It is known that the fluorescence emitted by “chiral molecules” can be distinguished. Using the expression “rotating”, right-rotating light (right-handed circularly polarized light) and left-rotating light (left-handed circularly polarized light) are detected. Studies on circular polarization have just begun, and the details are not yet clear, but it is said that some bio-related organisms distinguish between them when performing life-related activities. It is also said that this light can be used to create a new type of display. We would like to develop materials that can efficiently emit circularly polarized light (for example, the molecules on the left side of Figure 1) and use them in applications in the future.
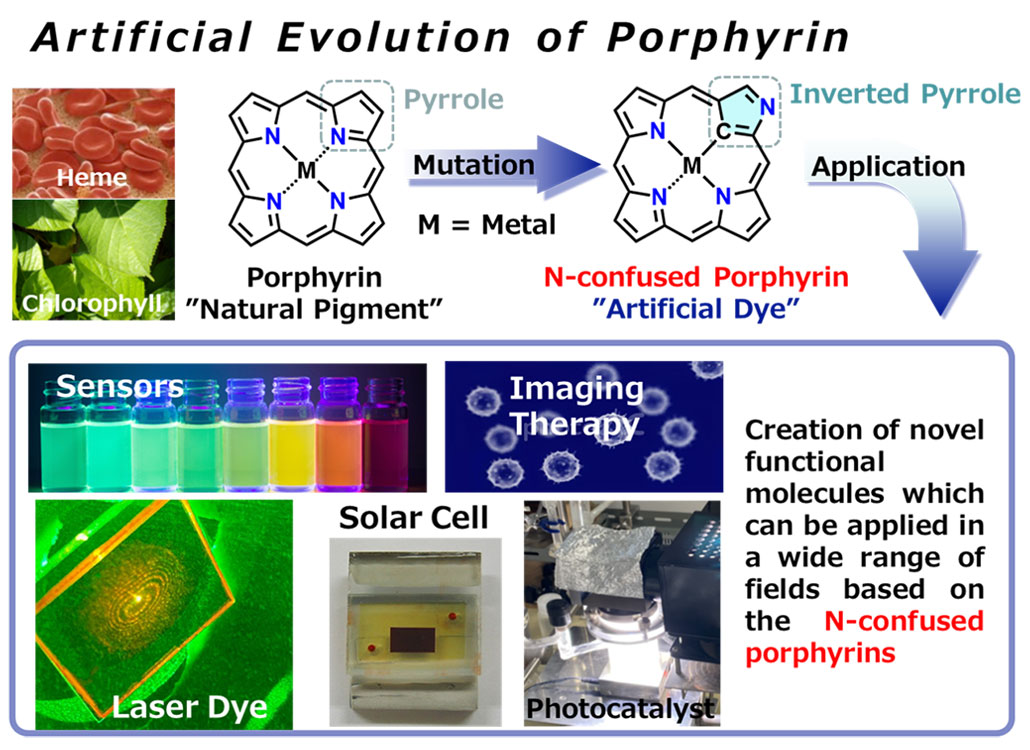
“Porphyrins” is a compound in which four nitrogen-containing five-membered ring subunits (so-called pyrrole) are alternately conjugated via methine carbon atoms and have been known as one of the essential natural pigments that are widely distributed in nature (e.g., chlorophylls present in plants and hemes in hemoglobin; see Figure 2). The specific tetrapyrrole macrocyclic structure can accommodate a variety of metal ions into the central ring system. The resulting metal porphyrin complexes have recently attracted attention for biomimetic catalysts, solar cells, and photo-therapeutic applications.
In an effort to seek a novel functionality of the porphyrins, we are exploring the direct mutation of the porphyrin skeleton by an “N-confusion modification,”; a design strategy that replaces a specifically inverted pyrrole among the tetrapyrrole scaffold. The systematic synthesis of the N-confused porphyrin analogues and their expanded/contracted systems with the different numbers/positions of the confused pyrroles induced the emergence of hitherto-unknown optical properties. For instance, for bioimaging applications, we have developed novel molecular dyes that absorb and emit deep near-infrared light to improve the permeability of intracellular light. In addition, photostable N-confused metalloporphyrin complexes were utilized as photosensitizing agents for photocatalytic water-splitting reactions and solar cell applications.
Environmental Geochemistry Laboratory: Atmospheric aerosols
General
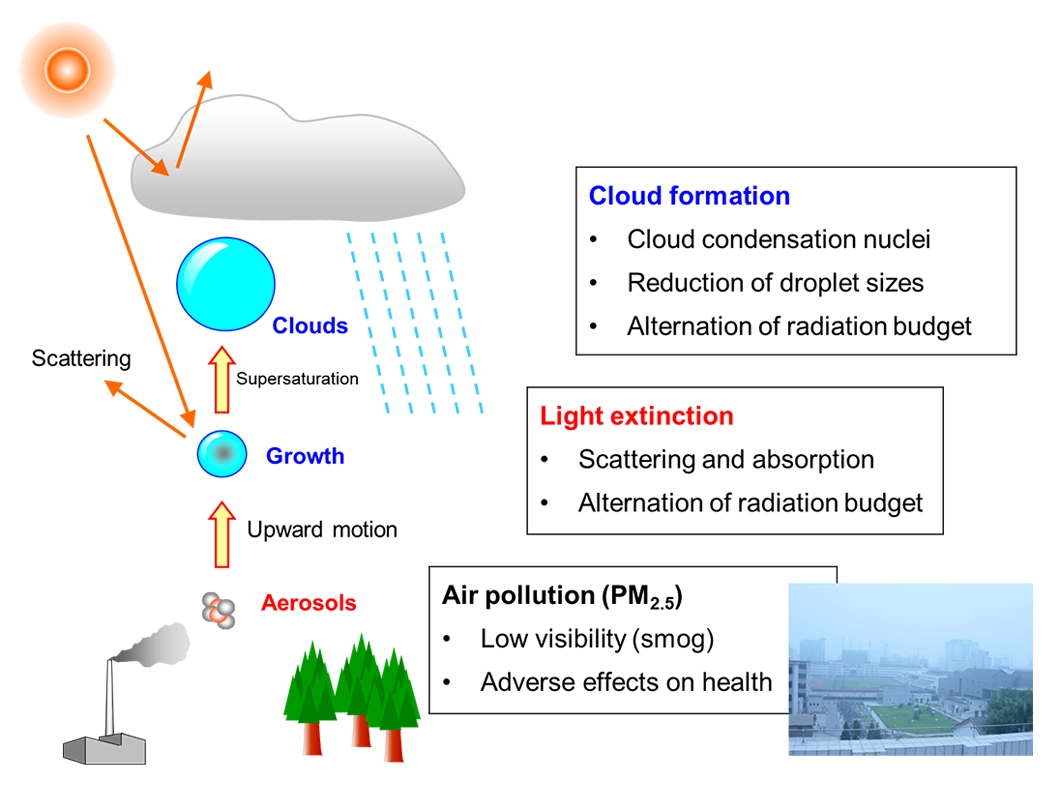
Aerosol is a collection of solid or liquid particles suspended in air. The sizes of aerosol particles range from a few nm to 100 μm, and the chemical compositions of aerosol particles show large variability depending on the emission sources and formation processes. Aerosols significantly affect air quality in urban air as they have adverse effects on human health. Aerosols can also affect the regional and global climate by altering the radiative balance of the Earth’s atmosphere (Fig. 1). The physical and chemical characterization of aerosol particles is important for improving our understanding of the environmental impacts of aerosols.
Development of a new aerosol composition analyzer
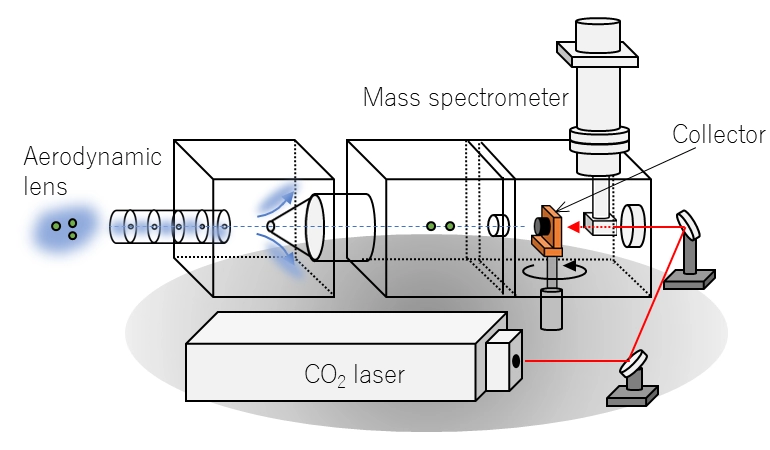
Online thermal desorption aerosol mass spectrometers (TDAMS) provide useful insights into the sources and formation processes of ambient aerosols. TDAMS instruments generally use a two-step detection scheme: aerosol particles are first collected and vaporized by thermochemical processes in a vacuum chamber, following which the evolved gaseous compounds are ionized and then detected. We have developed a new particle mass spectrometer (rTDMS) to separately quantify the mass concentrations of non-refractory and refractory sulfate compounds. A cup-shaped graphite collector coupled with a focused CO2 laser enables high desorption temperature (~1200 K). Laboratory experiments have demonstrated that the rTDMS can detect ion signals originating from refractory sulfate aerosols including K2SO4, Na2SO4, and MgSO4 (Kobayashi, Ide, and Takegawa, Aerosol Sci. Technol., 2021; Kobayashi and Takegawa, Atmos. Meas. Tech., 2022; Fig. 2).
Characterization of aircraft exhaust nanoparticles
Particle emissions from turbofan engines, which are commonly used in civil aviation, include the formation of non-volatile particles (mainly soot) during combustion processes and the nucleation and growth of volatile particles (mainly sulfate and organics) during plume expansion. We conducted field measurements of aerosols near a runway at Narita International Airport (NRT) in cooperation with National Institute for Environmental Studies. Our measurements have provided new insights into the characteristics of sub-10 nm particle emissions from in-use aircraft and the significance of jet engine lubrication oil as a source of aircraft exhaust nanoparticles at 10–30 nm (Fushimi et al., Atmos. Chem. Phys., 2019; Takegawa et al., Atmos. Chem. Phys., 2021).
Environmental impacts of aerosols
Schematic view of the new particle mass spectrometer (rTDMS)
Inorganic Chemistry Laboratory: Development and Analysis of Inorganic Materials Using Advanced Spectroscopy
Inorganic Chemistry Laboratory conducts the development of functional materials such as metal oxide clusters and complex metal oxides, and development of carbon dioxide capture and catalytic conversion, and elemental analysis of inorganic substances such as space and terrestrial substances. The followings are recent projects;
(1) Development of functional materials and their application as catalysts
It has been reported that metal oxide clusters composed of several metal oxide units exhibit strong acidity and basicity that cannot be expected from bulk metal oxides. Recently, we found that group 5 (Nb, Ta) metal oxide clusters showed unique base catalysis which was not observed in bulk metal oxides. Our laboratory aims to elucidate the base catalytic properties of metal oxide clusters at the atomic level and develop new base catalytic reactions that cannot be achieved with bulk metal oxides. In addition, we are also developing novel “vibro-catalytic” reaction systems using vibrational energy, which is unused energy, and low-energy material conversion technology using composite metal oxides with unique geometric and electronic structures.
(2) Development of new carbon dioxide capture/conversion technology
In order to achieve a carbon-neutral society by 2050, there is an urgent need to develop technologies for carbon dioxide capture and reusable. In order to solve these problems, our laboratory has developed a new Direct Air Capture technology using liquid-solid phase separation and a solid adsorbent with surface modification. In addition, we aim to develop new catalytic conversion of carbon dioxide using basic metal oxide clusters which can activate carbon dioxide.
(3) Operando measurement using synchrotron spectroscopy
Elucidation of the dynamic behaviors of the geometric and electronic structures of functional materials such as catalysts is important for understanding their functions. X-ray absorption spectroscopy using a synchrotron radiation facility can observe the electronic state of a target element and its surrounding local structure within milliseconds to several seconds. So, it is possible to obtain information on the state change of the target element in situ. Taking advantage of this feature, we aim to elucidate the synthesis mechanism and catalysis by operando measurements that combine X-ray absorption spectroscopy and other measurement techniques and to obtain design guidelines for the development of next-generation functional materials.
(4) Analysis using radiochemical methods
Activation analysis using nuclear reactions induced by neurons and photons can determine non-destructively trace elemental abundances in solid sample. We analyze environmental, geochemical, and cosmochemical samples with this analytical method to obtain a lot of information. In addition, natural and artificial radionuclides in these samples are determined using γ-ray spectrometry and accelerator mass spectrometry to study nuclear phenomena in nature and environmental behavior of radionuclides. We also develop elemental and isotopic analytical methods for these studies. We decipher the past and predict the future by chemical compositions.
Isotope Chemistry Laboratory: Development of Nanomaterials by Applying Radioisotopes(Ris)
In the Laboratory of Isotope Chemistry, radioisotopes (RIs) are applied for developing functional nanomaterials. We are now developing iron oxide nanoparticle photocatalysts with environmental purification, conductive vanadate glass as electrodes in secondary batteries, and metal-encapsulated fullerenes for drug delivery. In order to improve the properties of these materials, we are conducting research using 57Fe-Mössbauer spectroscopy using γ rays, which is a kind of radiation, and germanium (Ge) semiconductor detectors that can measure the types of RIs and their radioactivity. Two examples of research are introduced below.
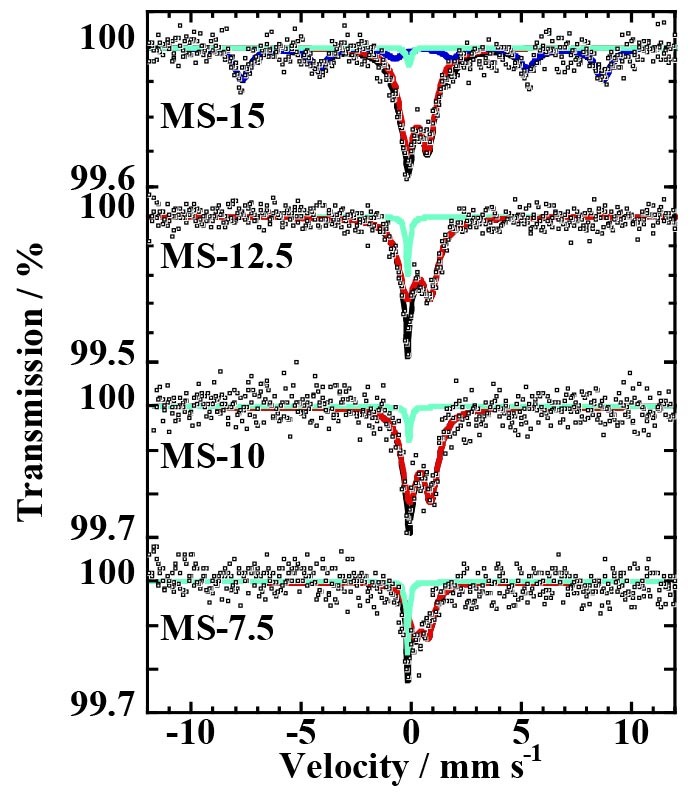
<Examle 1> Development of environmentally-purifying photocatalysts containing iron oxide nanoparticles and the structural analysis by Mössbauer spectroscopy
<Examle 2> Development of endohedral metallofullerenes with radioactive lanthanides as drug delivery agents